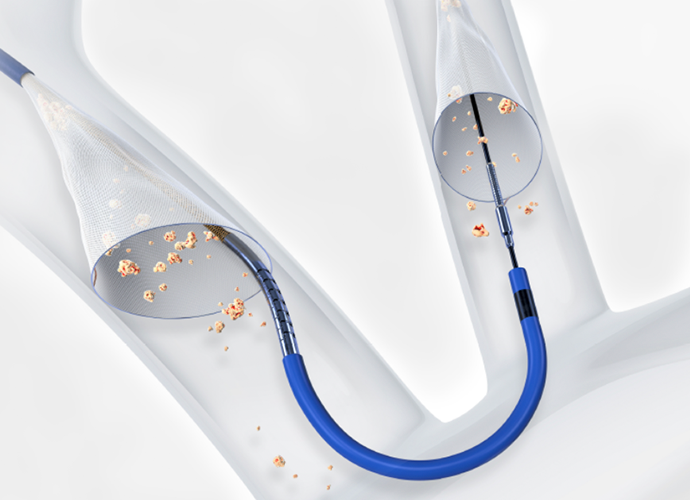
Minimize the risk of stroke during your TAVR procedure
If you have severe aortic stenosis, your heart team may recommend transcatheter aortic valve replacement (TAVR) to treat the condition. During the aortic valve replacement procedure, pieces of the calcified heart valve, or other debris, can break loose and travel through the arteries toward the brain. This material is called embolic debris. If left unaddressed, the debris could cause blockages, potentially leading to stroke.
To help protect patients from the risk of a stroke during TAVR, medical centers are beginning to offer Protected TAVR using the SENTINELTM Cerebral Protection System (CPS).
Hear OhioHealth Riverside healthcare providers talk about aortic stenosis and treatment options to help minimize your risk of stroke during TAVR in this Mended Hearts webinar, sponsored by Boston Scientific.
As with any medical device, there are risks associated with use of the SENTINEL Cerebral Protection System. These risks include but are not limited to: infection, blood vessel injury, stroke, death.
Issues specific to the use of the SENTINEL have been reported in a small number of patients and typically went away in 30 days. The use of SENTINEL during yourprocedure is solely at the discretion of your doctor.
1 Source: SENTINEL Clinical Trial